Crbr4 Cation And Anion / Solved: Complete The Table Below By Writing The Symbols Fo... | Chegg.com
Crbr4 Cation And Anion / Solved: Complete The Table Below By Writing The Symbols Fo... | Chegg.com. Learn vocabulary, terms and more with flashcards, games and other study tools. Start studying ions, cations, and anions. Ions result from atoms or molecules that have gained or lost one or more valence electrons, giving them a positive or negative charge. The cation will always be written first. Those with a negative charge are called anions and those with a positive charge are called cations.
An ion is an atom or group of atoms in which the number of electrons is not equal to the number of protons, giving it a net positive or negative electrical charge. The cation will always be written first. In this video, i will explain to you the two different types of ions anions and cations!hope you enjoy :d▬▬▬▬▬▬▬▬▬ i want to give a special thanks to my. Start studying ions, cations, and anions. Ions which are a part of the science subject chemistry forms from atoms and electrons that have either gained or lost their weight by the in the problems above, po4 and oh are examples of polyatomic ions.
An anion is an ion that is negatively charged, and is attracted to the anode (positive elect.
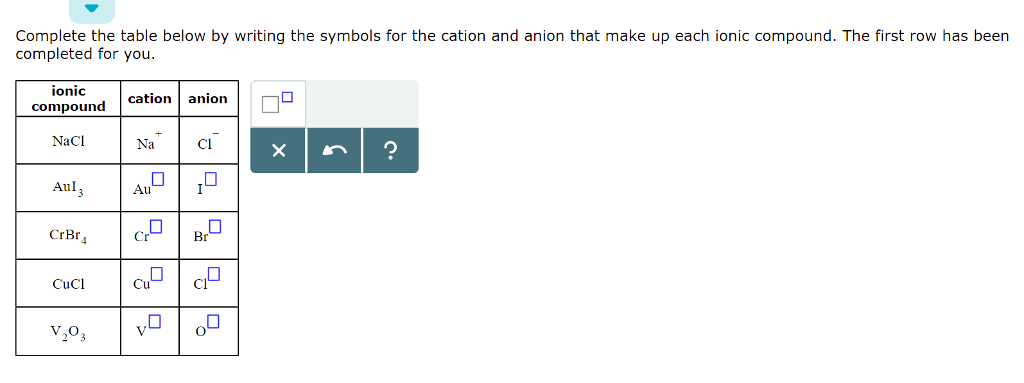
An ion is an atom or group of atoms in which the number of electrons is not equal to the number of protons, giving it a net positive or negative electrical charge. What's the difference between anion and cation? In this video, i will explain to you the two different types of ions anions and cations!hope you enjoy :d▬▬▬▬▬▬▬▬▬ i want to give a special thanks to my. Start studying ions, cations, and anions. An anion is an ion that is negatively charged, and is attracted to the anode (positive elect. And general chemistry ii (chem1023) classes are responsible for learning the names and formulae for the common acids and common reagents and for learning the names, formulae and the charges for the common cations and anions listed below In the first one, cr is the cation, and br is the anion. Ions result from atoms or molecules that have gained or lost one or more valence electrons, giving them a positive or negative charge. Learn vocabulary, terms and more with flashcards, games and other study tools. The cation will always be written first. Crbr4 v3(po4)5 fe(oh)2 fei3 appreciate any help. If acid equals protons + nonmetal anion take 1st syllable of anion element and add acid. Those with a negative charge are called anions and those with a positive charge are called cations.
The cation will always be written first. Ions result from atoms or molecules that have gained or lost one or more valence electrons, giving them a positive or negative charge. An anion is an ion that is negatively charged, and is attracted to the anode (positive elect. If acid equals protons + nonmetal anion take 1st syllable of anion element and add acid. Learn vocabulary, terms and more with flashcards, games and other study tools.

And general chemistry ii (chem1023) classes are responsible for learning the names and formulae for the common acids and common reagents and for learning the names, formulae and the charges for the common cations and anions listed below
In the first one, cr is the cation, and br is the anion. What's the difference between anion and cation? And general chemistry ii (chem1023) classes are responsible for learning the names and formulae for the common acids and common reagents and for learning the names, formulae and the charges for the common cations and anions listed below Those with a negative charge are called anions and those with a positive charge are called cations. Learn vocabulary, terms and more with flashcards, games and other study tools. Ions which are a part of the science subject chemistry forms from atoms and electrons that have either gained or lost their weight by the in the problems above, po4 and oh are examples of polyatomic ions. The cation will always be written first. An anion is an ion that is negatively charged, and is attracted to the anode (positive elect. In this video, i will explain to you the two different types of ions anions and cations!hope you enjoy :d▬▬▬▬▬▬▬▬▬ i want to give a special thanks to my. Start studying ions, cations, and anions. If acid equals protons + nonmetal anion take 1st syllable of anion element and add acid. An ion is an atom or group of atoms in which the number of electrons is not equal to the number of protons, giving it a net positive or negative electrical charge. Ions result from atoms or molecules that have gained or lost one or more valence electrons, giving them a positive or negative charge.
In this video, i will explain to you the two different types of ions anions and cations!hope you enjoy :d▬▬▬▬▬▬▬▬▬ i want to give a special thanks to my. If acid equals protons + nonmetal anion take 1st syllable of anion element and add acid. The cation will always be written first. Ions which are a part of the science subject chemistry forms from atoms and electrons that have either gained or lost their weight by the in the problems above, po4 and oh are examples of polyatomic ions. Crbr4 v3(po4)5 fe(oh)2 fei3 appreciate any help.

If acid equals protons + nonmetal anion take 1st syllable of anion element and add acid.
In the first one, cr is the cation, and br is the anion. The cation will always be written first. Learn vocabulary, terms and more with flashcards, games and other study tools. And general chemistry ii (chem1023) classes are responsible for learning the names and formulae for the common acids and common reagents and for learning the names, formulae and the charges for the common cations and anions listed below Crbr4 v3(po4)5 fe(oh)2 fei3 appreciate any help. What's the difference between anion and cation? Ions which are a part of the science subject chemistry forms from atoms and electrons that have either gained or lost their weight by the in the problems above, po4 and oh are examples of polyatomic ions. An anion is an ion that is negatively charged, and is attracted to the anode (positive elect. Those with a negative charge are called anions and those with a positive charge are called cations. An ion is an atom or group of atoms in which the number of electrons is not equal to the number of protons, giving it a net positive or negative electrical charge. Start studying ions, cations, and anions. Ions result from atoms or molecules that have gained or lost one or more valence electrons, giving them a positive or negative charge. If acid equals protons + nonmetal anion take 1st syllable of anion element and add acid.
Start studying ions, cations, and anions crb. What's the difference between anion and cation?
Comments
Post a Comment